
Even if the material's not ordinary, you cannot have 2 times 10 to the -19 coulombs.
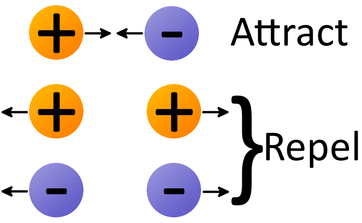
So 2 times 10 to the -19 coulombs is not possible, you cannot have that charge on anything. And since all these charge transfers are associated with transfers of electrons, it means that all charges that you can get on an object must be a multiple of this number right here. Now, the electron charge is -1.6 times 10 to the -19 coulombs. Whether it's a positive charge or a negative charge, always the electrons that are moving.Īlright. It's not the protons that are moving, it's always the electrons. And that's a very important thing for you to understand how that works. Whenever you've got a positive charge it means you've got too few electrons. So now, whenever you have a negative charge, it means you have too many electrons. And that means that fur is giving its electrons up to the amber. Now, along the same lines, if you rubbed fur on amber, the amber's going to get a negative charge. So you think well, jeez, if you've got positive charge you got too many protons. And that's something that sometimes is confusing to people especially since you know that there's all these protons business, right? And they've got positive charge. So that means that the glass has less electrons, still got the same number of protons, so that means that now it has a positive charge.

So instead of that this silk strips electrons away from the glass. So the silk isn't giving itself up to the glass. Wherever the protons are, that's where the silk is. Well, how does it do that? Now people might think the silk gives up protons to the glass but that's not what happens. And that means that when I rub silk on glass, the glass gets a positive charge. So electrons have a a negative charge, negative charge. So there is a certain type of particle that will be transferred from one object to another when we have charging going on.Īlright. But later experiments at the end of the 19th century showed that electric charge in ordinary materials is accomplished by the transfer of electrons. Now way long ago when people were first discovering electrofaction they had no idea what was causing it. Let's talk about electrons and quantization of electric charge.


 0 kommentar(er)
0 kommentar(er)
